
1b) via the Bernoulli equation (pressure gradient in mmHg = 4
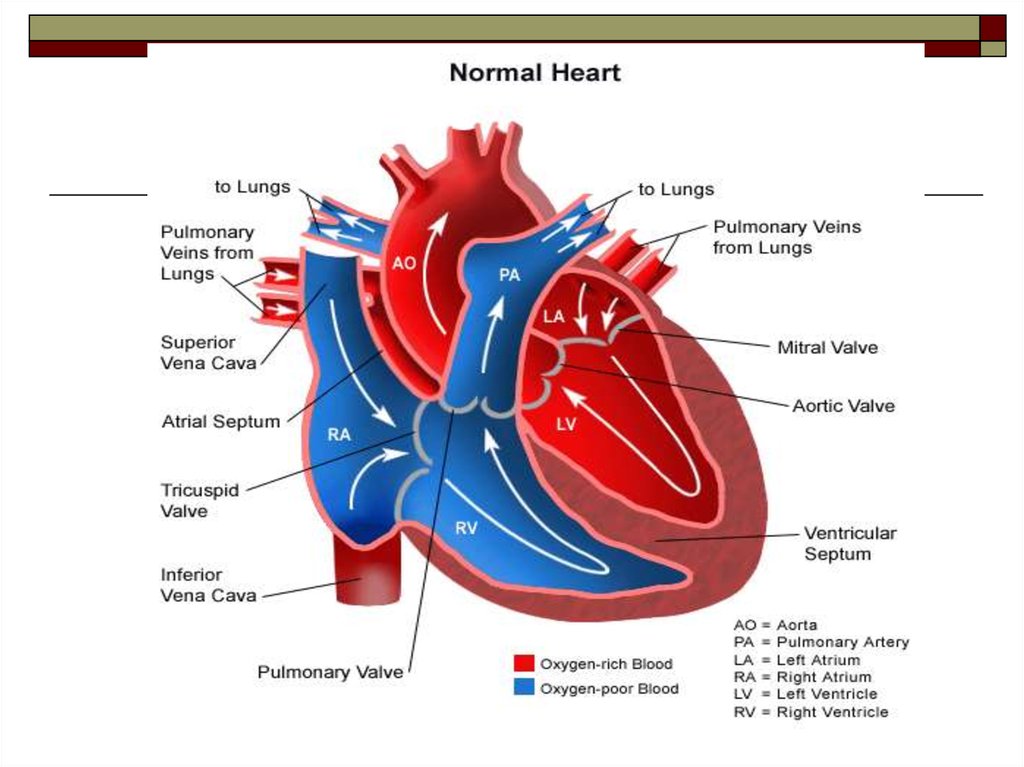
The systolic/diastolic pressure gradients between aorta and pulmonary artery of 7/2 mmHg, calculated from systolic/diastolic peak velocities across the PDA (Fig. 1d, e, f) and particle traces (see Additional file 1) originating from the PDA, demonstrated biphasic left-to-right shunting without Eisenmenger’s syndrome. 1c) during the cardiac cycle, as well as streamlines (Fig. Time-course of PDA maximal velocity (Fig. Multi-planar reformatted anatomical phase contrast images demonstrated a dilatated main pulmonary artery (cross-sectional area, 15.8 cm 2), and a tubular ductus arteriosus 24 mm in length and 104 mm 2 in cross-sectional area connecting the proximal descending aorta to the left pulmonary artery near its origin (Fig.
#BLOOD FLOW SOFTWARE#
Velocity fields were calculated, visualized and analyzed by dedicated prototype software (4D Flow V2.4, Siemens Healthcare, Erlangen, Germany).
#BLOOD FLOW FREE#
MR 4D flow data were acquired in free breathing, covering the heart, pulmonary artery and aorta with gapless slices of a retrospectively ECG-gated, segmented, two-dimensional spoiled gradient-echo-based cine phase-contrast sequence with three-directional velocity encoding in RVOT orientation. Left (LA) and right (RA) atrial volumes (maximum volume, LA: 39 mL/m 2, RA: 65 mL/m 2 minimum volume, LA: 19 mL/m 2, RA: 30 mL/m 2) were within normal ranges. Volumetric evaluation revealed enlarged left (LV) and right (RV) ventricular volumes (end-diastolic volume, LV: 126 mL/m 2, RV: 121 mL/m 2 end-systolic volume, LV: 55 mL/m 2, RV: 70 mL/m 2 ejection fraction, LV: 56 %, RV: 42 %), normal left (65 g/m 2) and increased right ventricular mass (61 g/m 2). Cardiac function was assessed by cine steady-state free precession real-time imaging during free breathing. To approach these issues, pulmonary arterial blood flow patterns were analyzed in a patient with PDA and PAH.Ī 21-year-old male patient (Table 1) with PDA and PAH (mPAP, 75 mmHg systolic/diastolic pulmonary arterial pressures, 102/58 mmHg systolic/diastolic systemic arterial pressures, 112/67 mmHg pulmonary arterial wedge pressure, 9 mmHg right atrial pressure, 7 mmHg cardiac index, 3.4 L/min/m 2, pulmonary vascular resistance, 11.5 Wood units) was referred for 3 T cardiac magnetic resonance (MR) function and 4D flow imaging (Magnetom Trio, Siemens Healthcare, Erlangen, Germany) after right heart catheterization (RHC) within a prospective study approved by the local ethical review board ( Identifier NCT01725763). Potentially, it might affect the presence of a vortex per se, the vortex location, the rotational direction, and also the relationship between vortex duration and mPAP. Pulmonary arterial hypertension (PAH) is a complication in patients with patent ductus arteriosus (PDA), and the retrograde blood flow in the main pulmonary artery caused by left-to-right shunting via the PDA might modify flow patterns typically observed in PH. Typically, vortex formation takes place at the posterior wall of the main pulmonary artery, and the vortex rotates in a clockwise direction when viewed in the right ventricular outflow tract (RVOT) orientation. In patients with pulmonary hypertension (PH) without abnormal aortic-to-pulmonary shunting, PH is associated with vortical blood flow along the main pulmonary artery, whose duration is linearly related to elevated mean pulmonary arterial pressure (mPAP).
This case indicates that PH-associated vortex rotation is not restricted to clockwise direction, and that vortex-based estimation of elevated mPAP might also be feasible in patients with PAH and PDA. Even though rotating counter-clockwise, duration of vortical flow translated into correct non-invasive mPAP estimate.

PH-associated vortical blood flow, which is typically observed rotating in a clockwise direction when viewed in right ventricular outflow tract orientation, was found nested in PDA left-to-right shunting. The present case analyzes the effect of a patent ductus arteriosus (PDA) on pulmonary artery flow patterns in PAH (mPAP from right heart catheterization, 75 mmHg). It remains to date not known, if this method is applicable in patients with pulmonary arterial hypertension (PAH) and abnormal aortic-to-pulmonary shunting. In patients with pulmonary hypertension (PH), duration of vortical blood flow along the main pulmonary artery enables estimation of the mean pulmonary arterial pressure (mPAP) non-invasively.


 0 kommentar(er)
0 kommentar(er)
